Home > About Us > Sustainability Management > Sustainability Report > Sustainability Report 2010 > Contributing to the Environment through R&D
 Contributing to the Environment through R&D
Contributing to the Environment through R&D
| We continue to develop new products and technologies across a wide range of fields with the aim of making a unique contribution to the global environment. |
| The Kobe Steel Group actively promotes the development of environmentally friendly products and technologies in an effort to create and boost sales of unique "Only One" Kobelco products. The following section outlines some of our latest research results, based on our technical expertise in the fields of materials and machinery. |
Joining technology for dissimilar metals designed to speed up automotive lightweighting
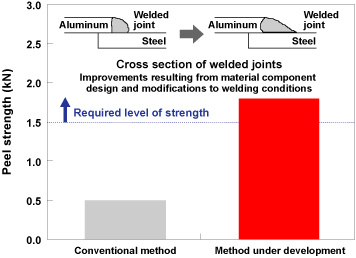
| Kobe Steel continues to develop a range of aluminum auto parts in order to help improve fuel consumption and reduce CO2 emissions, by reducing vehicle weight. As the development of aluminum panels for hoods and doors in particular would have a huge impact on vehicle weight, we are focusing on material and forming technology in an effort to expand applications (Figure 1). In order to use aluminum alloy materials for auto parts, it is essential to be able to join such parts to surrounding steel components. The process of joining dissimilar metals such as aluminum alloy and steel has always been performed mechanically until now, using bolts or rivets. This approach poses problems however in terms of both productivity and cost. Kobe Steel has therefore promoted to develop a dissimilar metal joining method based on the highly productive welding process commonly used to assemble vehicles. Using conventional methods, the compound layer formed between the joining parts is too fragile to produce strong enough joints. Through initiatives such as using substances that eliminate weld inhibiting materials known as fluxes and modifying welding conditions however, we have managed to develop technology that achieves the same level of joint strength as if welding two pieces of aluminum together (Figures 2 and 3). Although we are still only at the stage of establishing basic joining technology at the moment, we intend to continue with research and development with an eye to achieving application on actual parts in the future. |
Figure 1. Parts that can be made from aluminum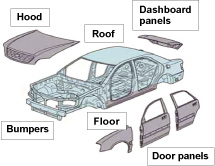 |
Figure 2. Example of aluminum alloy-steel welding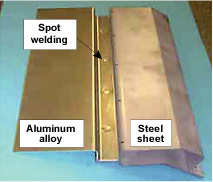 |
Figure 3. Comparison of weld strength of dissimilar metals using the conventional method and the method under development
Maintenance-free, corrosion-resistant steel designed to reduce the environmental impact of crude oil tankers
| The steady stream of major oil tanker disasters that seriously contaminated the environment up until the mid 2000s focused international attention on measures to prevent oil tank corrosion onboard tankers. Pitting (localized corrosion) on the steel materials used for oil tanks occurs as a result of strong acids, sulfur and other such substances contained in crude oil. Although there are international regulations in place requiring steel to have a corrosion-resistant coating, this is a labor-intensive process whilst tankers are docked and pitting can still occur and spread due to defects in the coating. As such, there has always been a need for a more efficient, more reliable solution. Kobe Steel has developed corrosion-resistant steel for crude oil tanks that is free from all high-environmental impact special additives and is effective in preventing pitting from spreading. The steel in question is currently being trialed onboard actual ship. During the development process, we managed to minimize corrosion by forming a stable protective coating on the surface of the steel and specifying its composition so as to prevent contact with sulfur, as well as harnessing compound constituents to increase pH levels within the pitted area and inhibit hydrogen evolution reactions (Figures 4 and 5). As this corrosion-resistant steel meets corrosion resistance requirements set out in international regulations that will be newly implemented and does not require maintenance, including protective coating and repairs to pitting during dock inspections, it will help to reduce environmental impact and save energy. As it is both extremely strong and exceptionally tough, it is also expected to make a substantial difference in terms of improving tanker safety. |
Figure 4. Corrosion resistant properties of steel under development (laboratory test results)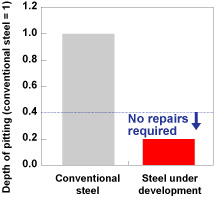 |
Figure 5. Test specimens after being dipped in strong acid solution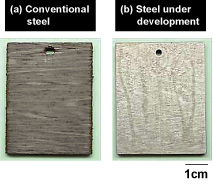 |
A compact hydrogen purification and supply process to promote the use of fuel cells
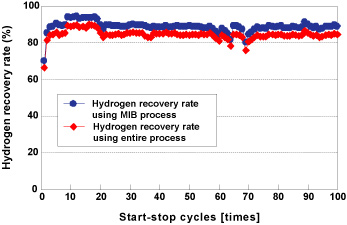
| If fuel cells are going to come into widespread use and help reduce CO2 emissions as hoped, it will be essential to establish the necessary hydrogen supply infrastructure. In order to create a hydrogen-powered society, we will need to come up with a system that is capable of efficiently purifying, storing and supplying hydrogen. In conjunction with the University of Tsukuba, Kobe Steel has developed the COA-MIB Process, a hydrogen purification and storage process that is compact, easy to start up and shut down and highly responsive to load variations (Figure 6). Using carbon monoxide (CO) selective adsorbents developed for the purpose of concentrating and recovering CO contained in byproduct gases from steelworks, the process completely removes CO mixed in with hydrogen produced from natural gas, making it possible to purify, store and supply hydrogen at high levels of purity using hydrogen storage alloys. This would eliminate the need for establishing a large-scale hydrogen supply network and, in practice, would enable pure hydrogen to be supplied to fuel cells at a high recovery rate of at least 85% on a DDS (daily start and stop) basis (Figure 7). We intend to step up development in the future and work on applications in areas such as natural energy generation, including small-scale hydrogen stations and solar cells, in an effort to combat climate change. |
Figure 6. Outline of the COA-MIB Process
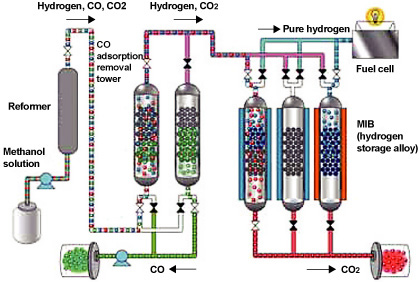
Figure 7. Hydrogen recovery rates based on a DSS cycle
High efficiency electrolytic ozone water production via conductive diamond-coated titanium electrodes
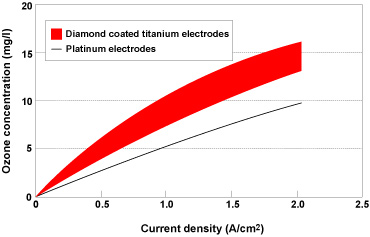
| Obtained from water through the process of electrolysis, electrolytic ozone water is a sterilizing cleaning fluid that has minimal impact on the environment and excellent oxidizing capabilities. It is widely used for purposes such as sterilizing air conditioning systems, eliminating odors, killing bacteria in medical institutions and processing food. Kobe Steel has developed electrodes for use in electrolytic ozone water production systems that are capable of producing electrolytic ozone water more efficiently than conventional platinum electrodes (Figure 8). In addition to regular diamond wafer coating technology, development also involved conductive diamond forming technology previously used in the electronic device sector. The electrodes consist of a conductive diamond coating around a base material made from titanium, which is highly resistant to corrosion, and enable electrolytic ozone water to be produced at concentrations 50-100% higher than platinum electrodes (Figure 9). As conductive diamond eliminates ion elution, the electrodes currently under development will also have a longer lifespan than platinum electrodes. We have already started supplying samples and are working on the development of commercial products in line with everyday needs and demand in the medical hygiene sector. |
Figure 8. Diamond-coated titanium electrodes

Figure 9. Comparison of electrolytic ozone water production efficiency using different electrodes